RedOaker News
 14 March 2014
14 March 2014
Rental/finance Costing (PDF Document) 29 January 2014
29 January 2014
New Finance Services (PDF Document) 11 March 2013
11 March 2013
New Winemaking Tools available 2013 Vintage!! (PDF Document) 11 September 2012
11 September 2012
Trial Sensory Results and Costing Data (PDF Document) April 2012
April 2012
Final 2011 McWilliams Trial Results (PDF Document) April 2012
April 2012
Toasting the Perfect Blend (PDF Document) 12th July 2009
12th July 2009
AWRI data (PDF Document) 3rd June 2009
3rd June 2009
RedOaker receives SA Great Commendation (PDF Document) 29 January 2014
29 January 2014
New Finance options (PDF Document) 11 March 2013
11 March 2013
New Advances and Applications for RedOaker (PDF Document)

The Potential of Dimethyl Silicone as a Gas Permeable Membrane for the Wine industry
Application: - The controlled ingress of oxygen into wine storage vessels in duplication of the oxidative function of oak barrels By Neal Palmer.
French professor and enologist Emile Peynaud (1984) discusses the role of oxygen relative to oak barrel maturation of wine and the manner in which an oak barrel acts as a physical mechanism...
![]() Download the pdf document
Download the pdf document
Or, see below for the online version
The Potential of Dimethyl Silicone as a Gas Permeable Membrane for the Wine industry
Application: - The controlled ingress of oxygen into wine storage vessels in duplication of the oxidative function of oak barrels By Neal Palmer.
French professor and enologist Emile Peynaud (1984) discusses the role of oxygen relative to oak barrel maturation of wine and the manner in which an oak barrel acts as a physical mechanism.
“In the case of barrel storage, contact with air and oxygen uptake occurs in three different ways, through the wood, via the wines surface due to the slight head space that always occurs in barrels, and finally during racking.
Oxygen permeation through the wood of sound oak casks is insignificant; it has been measured at 2-5ml/lt per year. It depends on thickness and type of wood; it is certainly greater in less close-grained wood and in smaller casks. In tuns where the wood is 5cm thick it is virtually nil.
Account should be taken to of the amount of oxygen surrendered by dry wood as it becomes impregnated after filling.
Uptake of oxygen via the surface is in the order of 15-20ml/lt per year whether the cask is put in bung-on-top position with topping at regular intervals or with the bung to one side.”
Wine stored in well made barrels tightly bunged are generally well protected from oxygen exposure. Moutounet et al. (1998) found the ullage oxygen content fell to about 1.8% within 2 months, CO2 content rising to about 28%.
Oxygen ingress into an oak barrel occurs principally as a result of typical cellaring procedures. Ribereau-Gayon (1931) also finds oxygen uptake to be 2-5ml/lt per year in tightly bunged full barrels increasing to 15-25ml/lt per year in barrels with typical ullage. The difference appears to relate to the development of ullage vacuum and the consequential increase in 02 ingress via micro flaws in the dryer staves above the wine surface. Partial drying of the wood above the ullage increases penetration (Vivas et al 2003). Singleton (1995) argues that most of the oxygen entering the lower wet staves must react with phenolic constituents within the stave before it can traverse.

2nd vintage barrel stave cross section
An Oak Barrel Alternative, re: Peynaud’s Theory
RedOaker (2002) duplicated the oak surface area to wine volume ratio of a 300lt new Hogshead barrel (employed as control) in a 500lt air tight stainless steel barrel with internally submersed oak staves. Free S02 and dissolved oxygen levels (using a “drop in” probe) were monitored over a period of 90 days.
The average discrepancy in dissolved oxygen levels between the containers was in the order of .05 ppm. The result suggested Emile Peynaud’s theory regarding the role of displaced oxygen from dry wood to avail oxygen to wine is well founded, as there were no other mechanisms to maintain dissolved oxygen levels within the sealed stainless steel barrel.
Tasting and sensory analysis indicated accelerated oak extraction in the sealed stainless steel barrel by comparison to the control. Approximately 60% of the oak staves were removed from the stainless steel barrel thereby depleting oxygen transfer and exposure potential of the container as a consequence.
To compensate for the decrease in available oxygen the stainless steel container was fitted with a highly permeable polyethylene membrane exposed to atmospheric conditions on one side, and a 10 litre argon purged ullage on the other employed to protect the free wine surface from excessive 02 exposure.
Accordingly the Stainless steel barrel recorded marginally elevated dissolved oxygen levels averaging a discrepancy of .15ppm higher than the control over a period of 12 months. The inert gas barrier effectively protected the free wine surface from acetobacter propagation during post malolactic maturation being periodically re-purged as a consequence of atmospheric exposure during sampling.
Polyethylene Maturation vessel
What appears certain is the controlled ingress of oxygen into a vessel storing wine, and the sequential oxidative reactions of wine compounds, is essential for what is generally regarded as beneficial to the sensory characteristics of the final wine product, particularly the more tannic red varietals. It has been shown the gas permeable properties of polyethylene containers lend themselves well to controlling oxygen transfer rates (otr’s) and typically produce fruit driven wines. The approach overcomes many difficulties associated with traditional oak barrels; particularly in regard to hygiene, cost, wine continuity and vessel longevity.
However, any polyethylene vessel will only permit oxygen ingress by virtue of its inherent physical properties and geometry. Wine stored within such a vessel will be exposed to a particular OTR irrespective of whether the given level of oxygen exposure is advantageous or detrimental to the winemaker’s requirements. Further, by virtue of the parameters ensuring a polyethylene container allows even relatively low rates of oxygen ingress, the result is often a container (volume dependent) with relatively thin and very flexible walls which are typically supported by means of a structural frame. This flexibility may interfere with the geometry and structural integrity of a thin walled vessel whilst under hydrostatic load.
A New Direction in Advance
Silicone rubbers are known to be several hundred times more permeable than polyethylene and therefore require a fraction of the surface area to permit equivalent levels of oxygen ingress.
This presents opportunities for the material to be employed as a gas permeable membrane where it may control an adequate rate of oxygen ingress into a wine vessel duplicating oxygen uptake rates associated with correctly functioning oak barrels. By this means an older oak barrel may have its ingressive oxidative functions effectively rejuvenated. Also other storage tanks (e.g. Stainless steel or polyethylene) stored in a controlled cellar environment may similarly exploit the advance. By introducing this type of membrane to a polyethylene vessel the necessity for the relatively thin walled low density material required to permit correct 02 permeation may be negated. Functional restrictions associated with such a vessel are also avoided thus enabling a substantially more robust and versatile vessel to effectively serve a dual purpose i.e. reductive 02 storage and 02 driven maturation.
The OTR of a Dimethyl Silicone Membrane A Method in Confirmation.
The oxygen permeation rate of a silicone membrane, in the first instance, was established by monitoring the ingress of oxygen into an airtight stainless steel cylinder incorporating an internal oxygen sensitive cell. The oxygen sensitive cell is in communication with a calibrated oxygen meter monitoring the 02 levels of a 50mm dia. cylinder having a capacity of 1000ml. The cylinder is effectively purged of oxygen and sealed using standard BSM type fittings with EPDM sealing orings. Oxygen ingress into the vessel was then monitored over a period of 7 days establishing a point of reference for the purposes of control.

Establishing control in the 1000ml test cell
With the integrity of the sealed vessel established, a silicone membrane was introduced as a sealing component at one end of the cylinder. Oxygen permeation was monitored over the same period of time and a comparison is made with results from those of the control, thus establishing the rate of oxygen ingress resultant of the introduced silicone membrane. The test was then repeated with a membrane of twice the thickness of the original to establish the effect thickness has upon the rate of oxygen ingress.

1000ml test cell with silicone Membrane in place
OTR Test Results
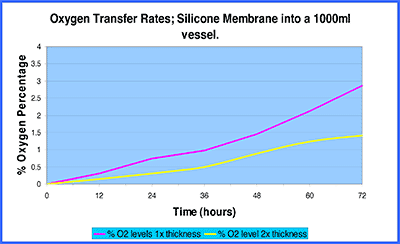
Comparative Silicone otr’s at 20deg.C and 1 surface
The table above illustrates the effect of a membranes thickness upon the ultimate transfer rate of oxygen through the membrane.
1x thickness membrane: 2.9% 02 ingress into 1000ml per 72 hrs
= 29ml 02 per 72 hrs
= 9.66ml 02 per 24 hrs
A Silicone Membrane for the Maturation of Wine In Application with RedOaker MS Series
Introducing the membrane described above (1x thickness) to a 1000lt RedOaker MS 1000 litre polyethylene storage container would in effect allow 3.53ml 02 per litre per year into the container. Membranes of varying thicknesses will be introduced as liquid tight sealing components controlling oxygen ingress into the inert gas purged ullage of the vessels.
The result is a series of various capacity containers that will effectively provide for the control of oxygen driven maturation by exposing wine to a wide range of controlled and adjustable levels of oxygen ingress in an otherwise identical manner and effect of a correctly functioning oak barrel.
In this way the robust, heavier walled and high density polyethylene MS Series vessels that otherwise lack the oxygen permeability to duplicate barrel rates of maturation, may allow oxygen ingress in the range of 3ml 02/lt per year to 30ml 02/lt per year using standard thickness membranes. Membranes may be configured of varying thicknesses thereby controlling oxygen ingress; particular attention is given to vessel capacity and ultimate 02 ingress per litre of each vessel in the MS Series range.
Protecting Free Surface Wine
The Use of Inert Gases
Either argon or an argon nitrogen mix is recommended for sparging/purging the ullage space. Argon is not the cheapest option, it makes up approximately 1% of our atmosphere and is therefore more expensive to refine by comparison with C02. Nevertheless it ultimately contributes around .01 cents/lt to the finished product when used in conjunction with the RedOaker MS Series units. In a correctly purged RedOaker MS Series unit it will take approximately 18months for the Argon to diffuse and dissipate through the silicone membrane.
The specific gravity of argon is close to CO2 (Ar =1.53 compared with CO2 = 2.264) but is only 38% as soluble in wine as CO2. Allen (1996b) noted that argon is the best gas to use for either alone or in a mixture of gases (see table). From the table it can be seen that the argon always had a positive effect on the colour, flavour/aroma and shelf life of the wine. Allen (1996b) did however not provide a mechanism to explain this phenomenon.
| Gas / Mix | Colour | Flavour / Aroma | Shelf Life (Degree of Oxidation) |
| Nitrogen | 70 | 50 | 50 |
| Ar | 95 | 90 | 90 |
| CO2 | 25 | 50 | 50 |
| N2 / CO2- 80:20 | 30 | 50 | 50 |
| Ar / CO2- 80:20 | 40 | 60 | 60 |
| Ar / N2- 80:20 | 90 | 90 | 90 |
| Ar / N2- 50:50 | 80 | 80 | 80 |
| Ar / N2- 20:80 | 70 | 70 | 70 |
(Relative Scaling of Effect compared to Oxygen, set to 0)
Note: Nitrogen, having a lower specific gravity than 0xygen, (sg .97 to 1.1@ 1 atm) is currently being tested during maturation with the gas permeable membrane allowing. Field trials using argon as a shielding gas have shown argon to be almost too effective in protecting the free wine surface, interfering with the potential of incoming 02 to dissolute into the wine mass via the free wine surface.
